A weak acid is an acid that only partially ionizes (dissociates) in water. This means that in solution, only a small fraction of the acid molecules donate protons (H⁺), resulting in a lower concentration of hydrogen ions compared to strong acids. Consequently, weak acids have a moderate pH and are less acidic than strong acids.
Characteristics of Weak Acids:
-
Partial Ionization:
-
Weak acids do not fully dissociate in water.
-
Instead, they establish an equilibrium between the undissociated acid molecules and the ions produced.
-
Example: Acetic acid
-
-
Lower Proton Concentration:
-
Because only a fraction of the molecules dissociate, the concentration of
H+ ions is relatively low.
-
-
Moderate pH:
-
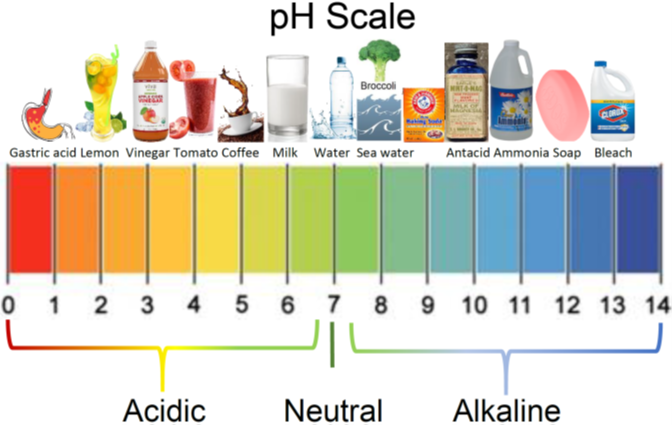
Weak acids typically have pH values between 3 and 6, depending on the concentration and strength of the acid.
-
-
Equilibrium Constant:
-
Weak acids have a smaller acid dissociation constant (Ka), indicating incomplete ionization.
-
Example: Ka for acetic acid (CH3COOH) is approximately
1.8×10−5
-
-
Examples:
-
Acetic acid ( CH3COOH): Found in vinegar.
-
Formic acid (HCOOH)= found in ant venom
-
Dissociation in Water:
The ionization of a weak acid in water is reversible and reaches equilibrium:
Where:
-
HA: Weak acid.
-
H3O+: Hydronium ion (proton donor in water).
-
A−: Conjugate base.